Latest DEVELOPMENTS
- On 26 November, the World Health Organization named the Omicron variant a "new variant of concern" after it had been identified a day earlier by scientists in South Africa. Omicron is spreading rapidly in South Africa, displacing Delta as the dominant strain among new cases, though it is unclear if Omicron originated in South Africa.
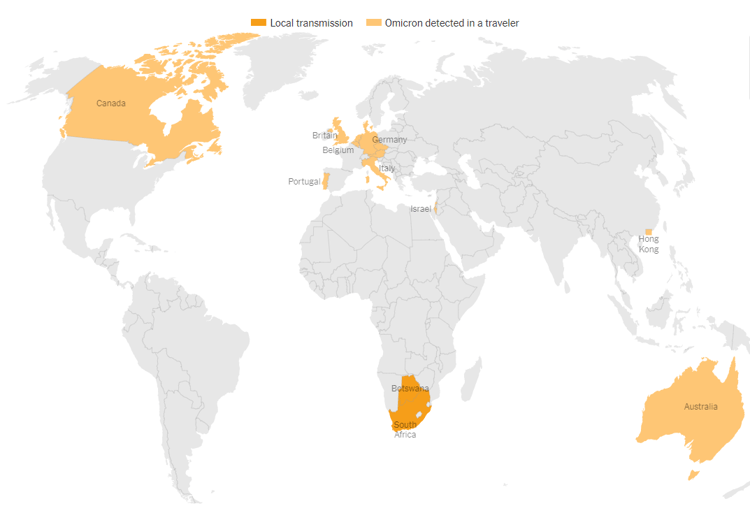
- Omicron has been detected in South Africa, Botswana, and in travelers to Australia, Belgium, Britain, Canada, Czech Republic, Denmark, Germany, Israel, Italy, the Netherlands, Portugal, and Hong Kong. This list will grow in the coming days and weeks.
- As a reaction to Omicron, many countries have banned flights from southern Africa, while several countries have significantly adjusted restrictions or banned all travel outright;
- Israel - banned all foreign travelers until at least 11 December. Returning nationals must quarantine
- Morocco - banned all incoming flights for at least the next two weeks.
- Japan - indefinite entry ban for all foreign nationals.
- Australia - flight suspension and entry ban for travelers and flights from southern Africa. Quarantine mandate and self-isolation requirement imposed for all international arrivals in Victoria, New South Wales, and Australian Capital Territory.
- While it is too soon to compare the symptoms and severity of Omicron to other iterations of the SARS-CoV-2 virus, Omicron has a number of mutations affecting the spike protein, raising concerns over possible immune evasion and increased transmissibility, as was seen with Delta, which currently comprises 99% of cases globally.
- Pfizer-BioNTech expects to receive lab data on the Omicron variant within the next two weeks. It says that they can adapt mRNA vaccine within six weeks and possibly ship initial batches within 100 days in event of escape variant.
- Moderna has told investors that it is evaluating a full booster dose of original vaccine, studying two multi-valent boosters, and plans on launching an Omicron-specific booster.
- Johnson and Johnson has announced that it is already testing its vaccine’s efficacy against the new variant of concern.
- Novavax is on pace to file for U.S. EUA before 2022. It has already applied for approval in the EU and Canada. Novavax is developing a new version of its vaccine and it will begin testing and manufacturing in the next few weeks.
The Global Guardian team is standing by to support your security and safety needs. To learn more about our corporate Duty of Care and Family Memberships, global tracking and intelligence platform (including COVID-19 updates and customized travel information), and medical evacuation capabilities, click below or call us at + 1 (703) 566-9463.